Veeva LIMS Spec Data records contain information about what samples to collect, what tests to run, and what evaluation criteria is used to determine if what’s tested is in or out of specification. Such criteria may be internal limits, specific to a market, or relate to a particular time point in a stability study. Spec Data records have associated Spec Data Mapping records, which match Materials and Countries to Test Definition records.
About Spec Data Mapping & References in Veeva LIMS
- Spec Data Mapping records match the Materials and Countries/ Organization on a Batch to Spec Data records. During Spec Execution, Veeva LIMS uses the Spec Data Mapping records with a status of Active on a custom lifecycle state or with the standard Active lifecycle state.
- Spec Data records reference Sample Plans (and their Sample Definitions, for use in Sample Pull actions) and contain Spec Data Criteria and Sample Actions.
- Sample Actions reference Sample Definitions in the Sample Plan associated with the Spec Data record. Sample Actions can be of different types: Pull Sample, Test Sample, Aliquoting Sample, Pool Sample.
- Spec Data Criteria reference Test Definitions and Results to Evaluate.
When products are registered with a governing health authority, a specification is established to use for quality control. The specification defines what tests will be done and the expected outcome. Each batch of a product is then tested and compared to the criteria set forth in the specification. This is done on finished products for the purpose of releasing to market, but also on raw materials, excipients, or active product ingredients (APIs) for the purpose of releasing for use in manufacturing.
About Spec Data Criteria
Each Spec Data contains a set of Spec Data Criteria, each targeting a specific Test Definition Result from a Test Definition in the selected lab protocol. The criteria defines the acceptable limits for the selected result, both descriptively and also as a formula.
Spec Data can be configured as Control specifications, providing early warning limits, or Release specifications, which would prevent batch release if failed.
Spec Data Change Analysis
Spec Data records are under version control via Veeva LIMS Change Analysis. Once these records enter the Effective lifecycle state, they cannot be edited. Instead, you must create a new version using the Create New Version action on the record.
Creating Spec Data
Before creating a Spec Data record, you should first have one or more Test Definitions and a Sample Plan in the Effective state type. You can create Spec Data using draft Test Definitions and Sample Plans, but you cannot approve it without first approving the draft Test Definition(s) and Sample Plan(s).
To create Spec Data:
- From the Spec Data tab, select Create.
- Choose Batch Release Spec Data or Stability Study Spec Data in the Select Spec Data Type drop-down.
- Click Continue.
- Enter a Name.
- Select the Sample Plan that will be used for this Spec Data.
- Click Save.
Vault validates that any calculated results are able to be completely calculated without cyclical references and populates the Effective Date field on the Spec Data when the record enters the Effective lifecycle state.
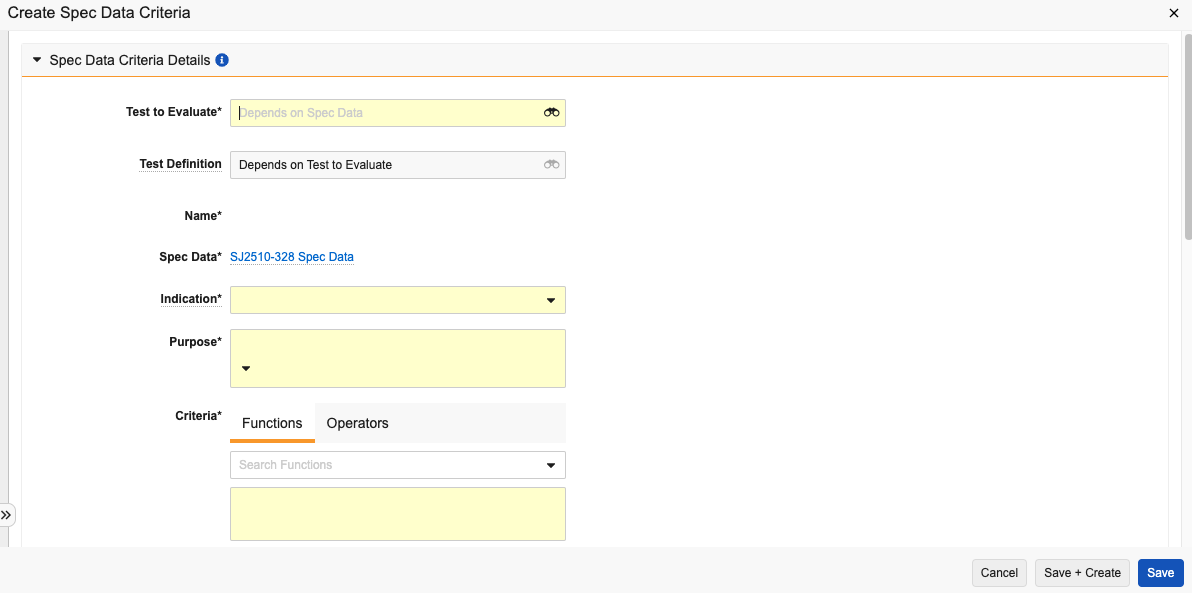
Creating Spec Data Criteria
Each Spec Data record has one or more criteria which define the detailed data limits for the Spec Data. Vault creates Lab Sample records for each criteria during sample initiation.
To create these criteria:
- In the Criteria to Evaluate section of a Spec Data record, click Create.
- Select a Test to Evaluate. This automatically populates the Test Definition field.
- Optional: Select a Test Definition Variation if applicable.
- Select a Result to Evaluate. This automatically populates the Result Object Type and Unit of Measure fields. It also populates the Variation Result Object Type field if you selected a Test Definition Variation.
- Enter a Name.
- Select an Indication. This determines how the criteria is represented during test execution and whether failing the criteria prevents spec conformance.
- Select a Purpose.
- Define a formula in the Criteria field that controls data limits based around the
$resulttoken, for example:$result < 0.5 - Enter a Criteria Description. This field serves as a human-readable explanation of the Criteria formula.
- Select a Spec Data Criteria Set.
- Click Save.
Additional Steps for Stability Study Spec Data Using Quantity Tracking
When creating Spec Data for a Stability Study that uses Quantity Tracking, it is necessary to create Spec Data Sample Actions in order to track quantities. To create one or more Spec Data Sample Actions, complete the following additional steps:
- On the Spec Data’s Record Details page, expand the Inventory Action section.
- Click Create.
- Enter a Name.
- Select a Sample Definition.
- Optional: Select a Unit. This will determine units for the Planned Quantity field.
- Enter the Planned Quantity.
- Click Save.
- Repeat the above steps as necessary for any additional Sample Actions.
Adding Planned Aliquoting to Spec Data
To add a Planned Aliquot to Spec Data, you must first ensure that the associated Sample Plan includes at least two sample definitions: one sample that is collected, and one that will be generated via aliquot. Then, add the Aliquot Action to the Spec Data record. To do this:
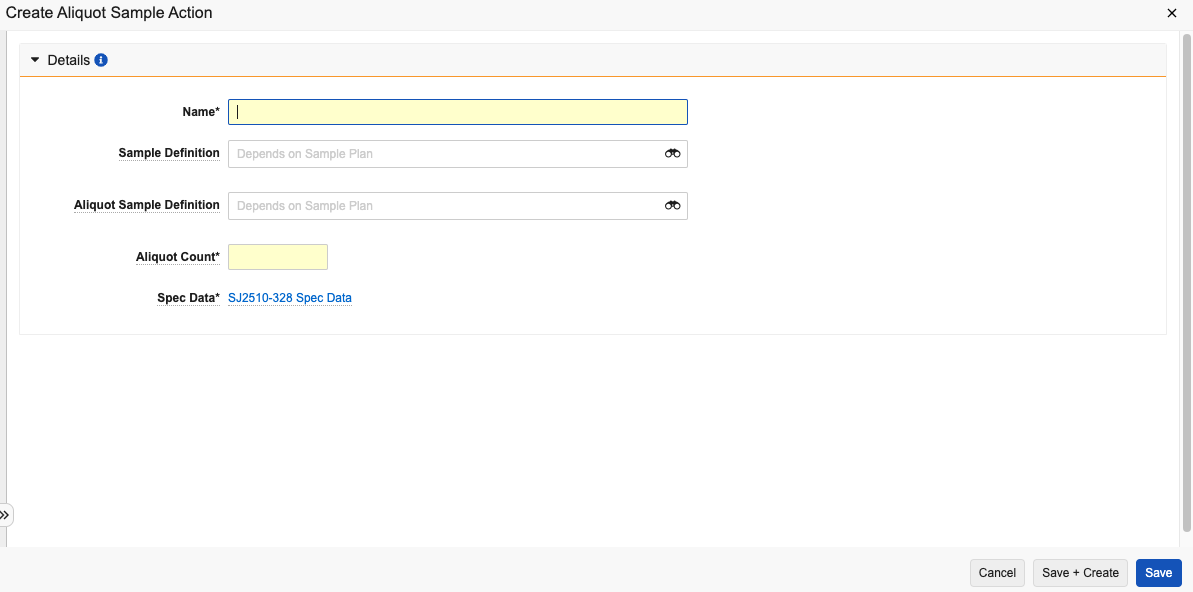
- In the Aliquots Actions section of a Spec Data record, select Create.
- Enter a Name.
- Select a Sample Definition. This represents the collected sample that should be pulled from storage to take the aliquot.
- Select an Aliquot Sample Definition. This represents the sample(s) created via aliquot.
- Enter an Aliquot Count.
- Click Save.
Note: Once a Spec Data record has been Approved, none of its standard fields can be edited, and standard relationships cannot be added or removed.
Related Permissions
To view and manage Spec Data, a user needs a permission set with View access to the Criteria field object control permission on the Spec Data Criteria object.